Faraday’s law
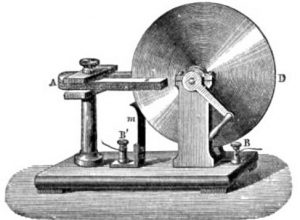
In the area of physics, Faraday's law permits the establishment of macroscopic phenomena of electromagnetic induction. This law investigates the study of magnetic fields, electromagnetism and electrochemistry. Based on Michael Faraday's work in 1831, it is a moderation law, which means that it describes effects that oppose its causes. Thanks to it, a set of laws capable of quantifying the amount of energy found in electrodes through their separation by means of electricity is proposed. Faraday's law is the fundamental law of magnetic induction according to which the electromotive force induced in a closed filiform circuit, is proportional to that derived with respect to time from the magnetic induction flux originated by this circuit.
What is Faraday's law?
Faraday's law alludes to any variation of flow within a closed circuit that causes an induced current that lasts the duration of the variation. The flow can vary either by variation of magnetic field lines or by variation of field strength.
About Faraday’s law
The first and second laws of Faraday are explained below.
Faraday’s first Law (First Law of Electrolysis)
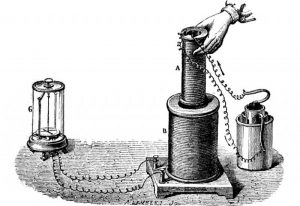
Faraday’s first law establishes a relationship between the mass of a substance released by electrolysis and the charge value that has passed through the electrolyte.
This law is formulated as follows: the mass of a substance that has been assigned during electrolysis on each electrode is directly proportional to the amount of charge that has passed through the electrolyte.
Formula
mass released = k (constant) · Q = k · I · t
where Q is the load in coulombs, I the intensity in amperes and t the time in seconds.
Faraday’s second law (second law of electrolysis)
Faraday’s second law establishes the dependence of the electrochemical equivalent of the weight substance and atomic valence. It is formulated as follows: the electrochemical equivalent of the substance will be proportional to its atomic weight, and inversely proportional to its valence called equivalent chemical substance. With this value, Faraday’s second law can be formulated differently: the electrochemical equivalents of the substance are proportional to their own chemical equivalents.
Formula
mass released = k (constant) · atomic weight / nº of oxidation
Faraday’s second law, like the first, derives directly from the nature of the ionic current in the solution.
History
The British Michael Faraday, one of the most important scientists for humanity despite not being well known, is the one who proposes Faraday’s law. Although he only had basic studies, he was very self-taught and interested in physics and chemistry from a very young age.
Thus, he began to experiment with what he knew, arriving at indications that led him to the discovery of electromagnetic induction and later, to carry out an experiment in which he achieves electrostatic induction, giving rise to the principle known as Faraday’s cage.
Being interested in both, electromagnetism and electrochemistry, he continues to make studies and notes regarding both subjects, when in 1834, he begins to formulate the so-called Faraday laws of electrolysis, which focus on calculating in a quantitative way, the amounts of energy deposited in the electrodes.
Examples where Faraday’s law applies
Here are some examples of objects that use Faraday’s law to fulfill their function.
Electric motors
For the creation of these gadgets, the notions of Faraday’s law are applied, since these gadgets can transform electrical energy into mechanical energy, offering an excellent performance with respect to chemical engines.
Induction glass-ceramic hobs
Induction hobs used in the kitchen are another invention based on the principles of Faraday’s law. These plates can detect when an object is placed on its surface and shakes it in one direction and the other through magnetic waves. Energy is absorbed and released in the form of heat.
Magnetic brakes
The functioning of magnetic brakes that can be found in the market are based on Faraday’s law. For example, when braking, a train energizes an electromagnet on board. This electromagnet goes on horseback on an elongated metal rail. The induced currents in the rail generate a force and thus the rail and the train repel each other.
How to cite this article?
Briceño V., Gabriela. (2019). Faraday’s law. Recovered on 24 February, 2024, de Euston96: https://www.euston96.com/en/faradays-law/