Electrolysis
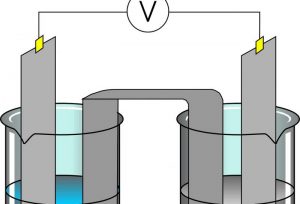
Electrolysis is a process by which the elements of a compound can be separated using electricity. Some substances such as salts and some metallic oxides are good conductors of electricity and suffer decomposition when they experience the passage of an electric current, these substances are called electrolytes, and this phenomenon is known as electrolysis. It is a process of oxidation and reduction that involves an increase in the free energy of the system that requires an external energy force. The reactions that occur in the electrolysis process are determined by energy laws.
What is electrolysis?
Electrolysis is the process by which the electric current passes through a substance to make a chemical change in it producing separation. The chemical change is one in which the substance loses or gains an electron and can be oxidation or reduction.
History of electrolysis
It was the British William Whewell, a great scientist, philosopher and author of numerous texts, who together with Michael Faraday in 1834 invented the term “electrolysis” to define the process of chemical decomposition of the galvanic current. At the beginning of the 19th century, this word was quickly introduced into chemistry, mineralogy and metallurgy through a series of experiments in which, by introducing electricity into a substance, different types of decompositions could arise, allowing us to know and understand the elements of that substance. In 1850 and 1860 medical experiments were made in North America and Europe using electrolysis.
What is it for?
Electro-metallurgy: many of the metals that are highly reactive, such as those belonging to group 1 and 2, are extracted from their matrices by electrolysis of their molten minerals. Metals such as sodium and magnesium are manufactured by their molten chloride electrolysis. Pure aluminum is obtained from a solution of its fused cryolite oxide and is used in the manufacture of some important substances such as hydrogen, chlorine, sodium hydroxide and oxygen. It is used in the production of aluminum, lithium, sodium, hydrochloric acid, potassium chloride, potassium hydrogen, and magnesium. It is used in saline solutions for the production of chlorine which is used in swimming pool water. Through electrolysis anodization it is possible to protect metals in corrosion state.
How it works
The electrical conductivity of substances is a displacement of the electrical charge through them. This displacement in charges can occur in two ways: through an electron flow, as usually happens in metals, and which are called first-species conductors, and through the movement of positive and negative ions, by a dissolution or a fluid ionic compound. This form of conductivity is known as ionic conductivity, also known as electrolytic conductivity, which is the conductivity of electrolytes that are second-species conductors. It is then, a process in which the passage of the electric current through a dissolution or a molten electrolyte results in an oxidation-reduction reaction, better known as redox.
Process
We can understand the process by looking at an example that involves the purification of a copper metal. This process will require an anode, a positively charged electrode and a cathode, a negatively charged electrode. In this case, an impure copper metal is the anode, while the pure copper metal is the cathode.
Application
Through the electrolysis process different compounds can be broken down into their elements as for example, hydrogen. It is also used to purify a metal such as copper. It is also used to coat some metals with another metal in order to beautify it or prevent corrosion. The metals that are usually deposited are gold, silver, chromium, tin and this process is called electroplating.
Water electrolysis
The electrolysis of water needs to function that the water is not in its pure state, it must have some concentrations of salts and some minerals; and direct current must be used during the process. It allows to obtain chemical elements, such as hydrogen and oxygen. It can be applied to fresh or saltwater, obtaining different results. From saltwater we will obtain chlorine and hydrogen in a gaseous state.
Electrolysis of sodium chloride
Chloride ions lose their electrons when they are transformed into chlorine atoms, which in turn form chlorine gas molecules. The anodic reaction that happens in this case is oxidation. These electrode reactions are called semi-reactions, and the overall reaction of sodium chloride electrolysis is: 2Na+ + 2Cl- → 2Na ° + Cl2.
How to cite this article?
Briceño V., Gabriela. (2019). Electrolysis. Recovered on 23 February, 2024, de Euston96: https://www.euston96.com/en/electrolysis/